Alicewilsonite-(YCe), a new member of the mckelveyite group of minerals, was officially discovered this February by researchers from the Canadian Museum of Nature and the Norwegian National History Museum. Formed thanks to Mont Saint-Hilaire's rare geological conditions, this crystal joins the extensive roster of minerals unearthed at the Mont St-Hilaire Quarry, some for the first time. One of several close look-alikes, this mineral was identified using high-resolution electron microprobe analysis and X-ray diffraction.
Despite its microscopic scale, this research has implications across disciplines, including medicine and the study of pollutants.
A new crystal identified at Mont Saint-Hilaire
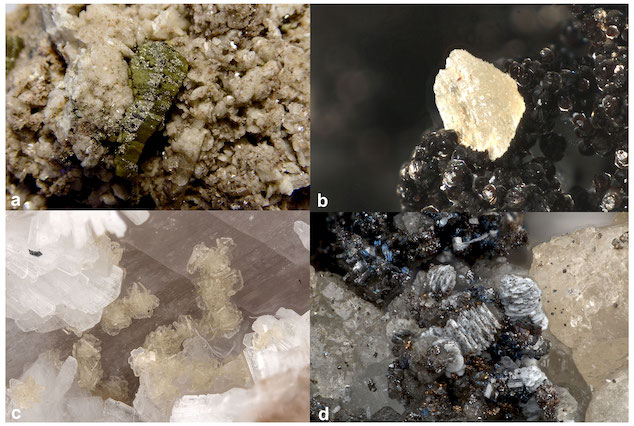
Four specimens of alicewilsonite-(YCe). It can be green (photo a), yellow (photo c), white (photos b, d), or some mix of the three, and very often forms on top of another mckelveyite group mineral (photo: Lykova et al., 2023)
Yet another new mineral has been discovered at the Gault Nature Reserve by Inna Lykova, Ralph Rowe, and Glenn Poirier of the University of Ottawa, Henrik Friis of the Natural History Museum of Oslo, Norway, and Kate Helwig of the Canadian Conservation Institute.1 Alicewilsonite-(YCe), named after Canadian geologist Alice Wilson (1881–1964), is a member of the mckelveyite family of crystals containing the rare earth elements yttrium and cerium. The specimen was originally thought to be a variation of a known mineral, donnayite-(Y). Further analysis of specimens collected at Gault and held at the Canadian Museum of Nature in Ottawa, however, revealed alicewilsonite to be a distinct crystal in February 2023. This makes it the 72nd mineral to be discovered for the first time here at Mont-Saint-Hilaire.
The mckelveyite crystals are known to be visually indistinguishable, with only small differences in their chemical makeup. So how did these scientists discover that alicewilsonite was something new?
How are minerals identified?
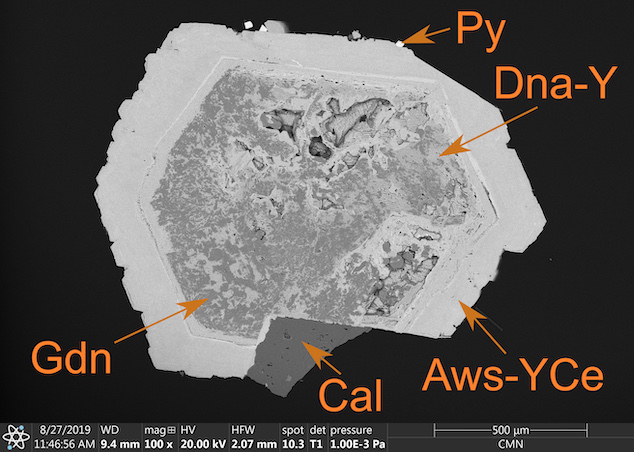
Alicewilsonite (Aws-YCe) crystal formed around a central column of donnayite (Dna-Y), with traces of gaidonnayite (Gdn), calcite (Cal), and pyrite (Py) also indicated (photo: Lykova et al., 2023)
Electron microprobe analysis is a technique used to identify the chemicals and elements in a compound2 —in this case, alicewilsonite. A beam of electrons is fired at the material, temporarily exciting its atomic constituents, and causing them to emit X-rays. These X-rays are unique to each element, allowing mineralogists to determine the chemical makeup of the material they are studying. This technique has a resolution of one micrometre (one-thousandth of a millimetre) and can distinguish all but the three smallest elements of the periodic table.
Electron microprobe analysis only identifies what a material is made of, however, not how its elemental building blocks fit together. Most minerals are crystalline, meaning their constituent molecules form a regular, repeating geometric lattice, like building blocks fitting together. A crystal is an agglomeration of these repeating molecules, starting with a “seed” and growing as it accumulates. To map the structure of a crystal, mineralogists use X-ray diffraction in a small-scale recreation of getting an X-ray at the doctor’s office. Flat facets within the crystal deflect X-rays more strongly, which scientists can use to calculate where the planes and lines of symmetry characteristic of a crystal lattice are.
Why does Gault have such a rich diversity of mineral species?

A display at McGill’s Redpath Museum of minerals unearthed from Mont-Saint-Hilaire (photo: Alex Tran)
Alicewilsonite is just one more among hundreds of minerals found at Gault Nature Reserve. Mont Saint-Hilaire is an alkaline complex, a rock formation rich in sodium (Na), potassium (K), and rare earth elements.4 When the mountain formed from an upwelling of magma 125 million years ago, this potassium- and sodium-rich magma formed an unusual environment in which the rare-earth elements brought up from the Earth’s core could form compounds that do not occur under other conditions. To date, 434 different minerals have been identified at Mont Saint-Hilaire, which is 9% of the 5000 species identified worldwide, and 72 of them were first found here. Most of these minerals come from Mont Saint-Hilaire Quarry, on the North-East side of the mountain, where a cut into the mountain gives miners, collectors, and scientists alike access to the valuable stone that makes up Mont Saint-Hilaire. Collections of mineral specimens found at Gault Nature Reserve can be found at McGill’s Redpath Museum in Montréal and the Canadian Museum of Nature in Ottawa.
Why do scientists study minerals and crystals?
Alicewilsonite is not found exclusively at Gault. Other specimens previously considered donnayite were reanalyzed and discovered to be this new mineral. These specimens come from other mines as close as Saint-Amable to as far as the Kola Peninsula of Russia and the Igaliko alkaline complex in Greenland. As mineralogy helps us understand how geographical formations occurred and under what conditions, this tells us that these three locations—in Quebec, Northern Russia, and Southern Greenland—may have formed under similar conditions, despite being thousands of kilometres apart.
But the study of crystal formation isn’t only important to geology. It tells us how different molecules accumulate and bond together in nature, which can be applied in chemistry, biology, medicine, meteorology, mineralogy, physics, and material and environmental sciences as well. Crystallization doesn’t only occur in rocks; one crystal every person relies on daily is hydroxyapatite (Ca5(PO4)3OH), the mineral that makes up 70% of the mass of human bones.5 Better understanding of complex crystal formation is an integral step on the path to being able to grow bone-like material in labs for medical transplants, for instance.6
Closer to the hearts of those at Gault Nature Reserve, studying how molecules fit together, attract each other, and interact with other materials gives us a better understanding of how pollutants move through and interact with our natural environment.7 Alkaline complex minerals form in unusual ways and studying how they crystallize broadens scientists’ knowledge of how molecules aggregate in general. This could help us understand the chemical processes that cause pollutants to accumulate in particular environments, and, hopefully, how best to eliminate or prevent them.

Wind River Stream (photo: Kylir Horton, 2023, CC BY 2.0)
Seonaid G. Newell-Macintosh
Field Operations Assistant
Gault Nature Reserve of McGill University
Learn more
- See a map of the different geological zones found at Gault Nature Reserve and when they formed
- Read about the mineral diversity of Mont Saint-Hilaire on our website
- Find a quick explanation of how the mountain formed
References
- Lykova, Inna et al. “Mckelveyite Group Minerals – Part 2: Alicewilsonite-(YCe), Na2Sr2YCe(CO3)6 ⋅ 3H2O, a New Species.” European Journal of Mineralogy 35, no. 1 (February 28, 2023): 143–55. Read the article.
- Reed, S. J. B. "Electron Microprobe Analysis and Scanning Electron Microscopy in Geology." Cambridge University Press, 2005.
- Perkins, Dexter. "Crystals and Crystallization." In Mineralogy, 2nd ed., 2022. Read the article. CC BY-NC-SA 4.0. Read the article.
- Canadian Museum of Nature. "Mineral Diversity at Mont Saint-Hilaire." YouTube video, 5:14. March 29, 2021. Watch the video.
- "Hydroxyapatite." In Wikipedia. July 2, 2023. Read the article.
- University of Washington News. "Crystals Form through a Variety of Paths, with Implications for Biological, Materials and Environmental Research." UW News (blog), August 3, 2015. Read the article.
- De Yoreo, James J. et al. “Crystallization by Particle Attachment in Synthetic, Biogenic, and Geologic Environments.” Science 349, no. 6247 (July 31, 2015): aaa6760. Read the article.
Header: A display at McGill’s Redpath Museum of minerals unearthed from Mont-Saint-Hilaire (photo: Alex Tran)

